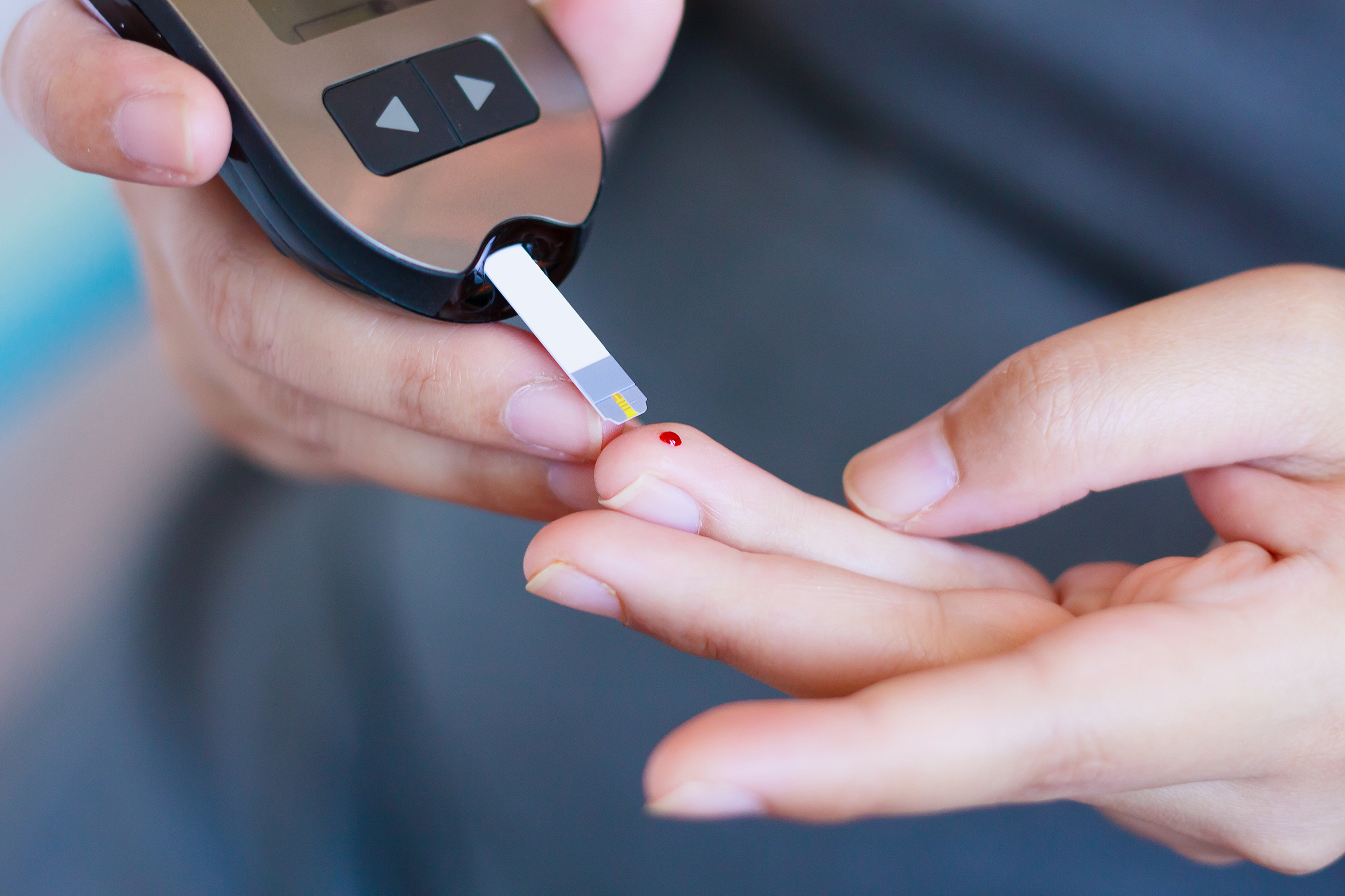
While diabetes is characterised by a deficiency of insulin, the opposite is the case in congenital hyperinsulinism: patients produce the hormone too frequently and in excessive quantities, even if they haven’t eaten any carbohydrates.
Since the function of insulin is to metabolise sugars, excess production of insulin leads to chronic hypoglycaemia. The brain, which devours vast quantities of energy, is perpetually undernourished.
The disorder can therefore lead to serious brain damage and even death in the worst cases. A team at the University of Geneva (UNIGE), Switzerland, supported by the Swiss National Science Foundation (SNSF) has succeeded in precisely describing the effects of a frequent genetic mutation in cases of congenital hyperinsulinism. This discovery, which has been published in Human Molecular Genetics, could pave the way for new therapies.
Congenital hyperinsulinism starts exerting its effects from birth. Although it is considered to be a rare disease, affecting roughly one in every 50,000 newborn babies, it may be underdiagnosed.
‘Unless you are looking for it, hypoglycaemia can easily go unnoticed in an infant,’ explained Pierre Maechler, a researcher at the Faculty Diabetes Centre from the Faculty of Medecine, UNIGE and the lead author of the study.
‘Without intervention it can rapidly take a dramatic course.’
The researchers focused on a genetic mutation known to be associated with hyperinsulinism. This gene produces a protein known as GDH, which instructs the pancreas to release insulin. It normally behaves differently once the level of blood glucose passes a certain threshold. Then GDH opens up to receive a molecule known as an accelerator that binds to it. In this way the protein moves into the active phase, which in turn sends a signal to the pancreas, causing it to produce more insulin.
In congenital hyperinsulinism the mutant gene causes the structure of the protein to change. The protein remains permanently receptive to the accelerator molecule, whatever the level of glucose in the blood. As a result, it constantly sends signals to the pancreas, telling it to release insulin, which it then does excessively.
This new study could pave the way for new treatments.
‘We can imagine developing a drug that inhibits the GDH accelerator by occupying the same site, which would reduce the production of insulin,’ Pierre Maechler said.